Important Safety Information
Contraindications
Apidra is contraindicated during episodes of hypoglycemia and in patients hypersensitive to Apidra or any of its excipients.
Warnings and Precautions
Insulin pens and needles must never be shared between patients, even if the needle is changed. Do NOT reuse needles.
Monitor blood glucose in all patients treated with insulin. Modify insulin regimens only under medical supervision. Changes in insulin regimen including, strength, manufacturer, type, injection site or method of administration may result in the need for a change in insulin dose or an adjustment in concomitant oral antidiabetic treatment. Dosing should be individualized based on patient characteristics and lifestyle.
Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis may result in hyperglycemia; sudden change in the injection site (to unaffected area) has been reported to result in hypoglycemia. Advise patients to rotate injection site to unaffected areas and closely monitor for hypoglycemia.
Hypoglycemia is the most common adverse reaction associated with insulins, including Apidra, and maybe life-threatening.
To avoid medication errors between APIDRA and other insulins, instruct patients to always check the insulin label before each injection.
All insulin products, including Apidra, can cause hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Use caution in patients who may be at risk for hypokalemia.
Severe life-threatening, generalized allergy, including anaphylaxis, can occur with insulin products, including APIDRA. If hypersensitivity reactions occur, discontinue APIDRA, treat per standard of care and monitor until symptoms and signs resolve.
Fluid retention and heart failure can occur with concomitant use of thiazolidinediones (TZDs) with insulin. Observe for signs and symptoms of heart failure. Consider dosage reduction or discontinuation of TZD if heart failure occurs.
Malfunction of the insulin pump or insulin infusion set or insulin degradation can rapidly lead to hyperglycemia and ketoacidosis. Patients using insulin infusion pump therapy must be trained to administer insulin by injection and have alternate insulin therapy available in case of pump failure.
Adverse Reactions
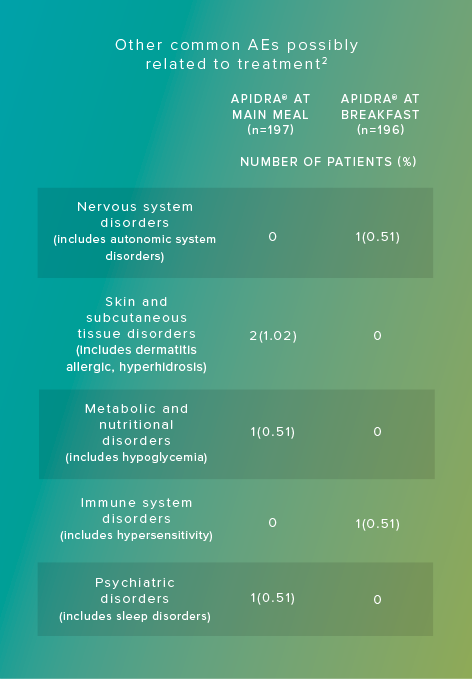
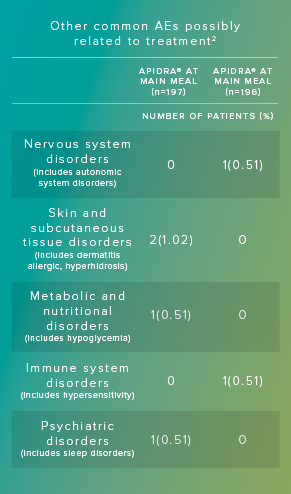
Adverse reactions commonly associated with Apidra include hypoglycemia, allergic reactions, injection site reactions, lipodystrophy, pruritus, rash, and weight gain.
Drug Interactions
Certain drugs may affect glucose metabolism, requiring insulin dose adjustment and close monitoring of blood glucose. The signs of hypoglycemia may be reduced in patients taking anti-adrenergic drugs (e.g., beta-blockers, clonidine, guanethidine, and reserpine).
Important Safety Information for Apidra® SoloStar®
Apidra SoloSTAR is a disposable single-patient-use prefilled insulin pen. To help ensure an accurate dose each time, patients should follow the steps in the Instruction Leaflet accompanying the pen; otherwise they may not get the correct amount of insulin, which may affect their blood glucose.
Important Safety Information for Apidra Use in Pump
Inform patients to replace the infusion sets (reservoir, tubing, and catheter) and the Apidra in the reservoir at least every 48 hours and select a new infusion site. Pump failure or insulin infusion set or insulin degradation can rapidly lead to hyperglycemia and ketoacidosis. Prompt identification and correction of the cause is necessary. Interim subcutaneous injections with APIDRA may be required. Patients using a pump must be trained to administer insulin by injection and have alternative insulin therapy available in case of pump system failure.
for Full Prescribing Information for Apidra.
for information on Sharps Medical Waste Disposal.
to learn more about Sanofi's commitment to fighting counterfeit drugs.
*Eligibility Restrictions & Offer Terms:
Insulins Valyou Savings Program:
Sanofi insulins included in this program are: ADMELOG® (insulin lispro injection) 100 Units/mL, TOUJEO® SoloStar® (insulin glargine injection) 300 Units/mL, TOUJEO® Max SoloStar® (insulin glargine injection) 300 Units/mL, LANTUS® (insulin glargine injection) 100 Units/mL, INSULIN GLARGINE injection 100 Units/mL (U-100) and APIDRA® (insulin glulisine injection) 100 Units/mL.
This offer is not valid for prescriptions covered by or submitted for reimbursement under Medicare, Medicaid, VA, DOD, TRICARE, similar federal or state programs, including any state pharmaceutical programs, or commercial/private insurance. Only people without prescription medication insurance can apply for this offer. Void where prohibited by law. For the duration of the program, eligible patients will pay $35 per 30 Day Supply. To pay $35 per 30 Day Supply, you must fill all your Sanofi Insulin prescriptions at the same time, together each month. Not valid for SOLIQUA 100/33 (insulin glargine and lixisenatide) injection 100 Units/mL and 33 mcg/mL. When using the Insulins Valyou Savings Card, prices are guaranteed for 12 consecutive monthly fills. The Insulins Valyou Savings Program applies to the cost of medication. There are other relevant costs associated with overall treatment. Sanofi reserves the right to rescind, revoke, terminate, or amend this offer, eligibility, and terms of use at any time without notice. Upon registration, patients will receive all program details.
Sanofi Copay Program:
This offer is not valid for prescriptions covered by or submitted for reimbursement under Medicare, Medicaid, VA, DOD, TRICARE, or similar federal or state programs including any state pharmaceutical assistance program. If you have an Affordable Care (Health Care Exchange) plan, you may still be qualified to receive and use this savings card. Please note: The Federal Employees Health Benefits (FEHB) Program is not a federal or state government healthcare program for purposes of the savings program. Void where prohibited by law.
-
Apidra: $0 copay with maximum savings up to $100 per pack up to 1 pack per fill.
Savings may vary depending on patients’ out-of-pocket costs. Upon registration, patients receive all program details. Sanofi US reserves the right to change the maximum cap amount, rescind, revoke or amend these programs without notice.
REFERENCES:
- Sheldon B, Russel-Jones D, Wright J. Diabetes Obes Metab. 2009;11:5-9.
- Becker RHA, Frick AD, Burger F, Potgieter JH, Scholtz H. Exp Clin Endocrinol Diabetes. 2005;13:435-443.
- Bolli GB, Luzio S, Marzotti S, et al. Diabetes Obes Metab. 2011;13:251-257.
- Heise T, Nosek L, Spitzer H, et al. Diabetes Obes Metab. 2007;9:746-753.
- Apidra® Prescribing Information.
- NovoLog® Prescribing Information.
- Humalog® Prescribing Information.
REFERENCES:
- AACE/ACE Consensus Statement. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2016 Executive Summary. T2D Algorithm, Executive Summary. Endocr Pract. 2016;22(No.1).
- Apidra® Prescribing Information.
- NovoLog® Prescribing Information.
- Humalog® Prescribing Information.
Indication and Usage for Lantus® (insulin glargine injection) 100 Units/mL
Indication and Use
Lantus is a long-acting human insulin analog indicated to improve glycemic control in adult and pediatric patients with diabetes mellitus.
Limitations of Use
Lantus is not recommended for the treatment of diabetic ketoacidosis.
Important Safety Information
Contraindications
Lantus is contraindicated during episodes of hypoglycemia and in patients with hypersensitivity to insulin glargine or any of the excipients in LANTUS.
Warnings and Precautions
Insulin pens, needles, or syringes must never be shared between patients. Do NOT reuse needles.
Monitor blood glucose in all patients treated with insulin. Modify insulin regimen only under medical supervision. Changes in insulin regimen including, strength, manufacturer, type, injection site or method of administration may result in the need for a change in insulin dose or an adjustment in concomitant drugs.
Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis may result in hyperglycemia; sudden change in the injection site (to unaffected area) has been reported to result in hypoglycemia. Advise patients to rotate injection site to unaffected areas and closely monitor for hypoglycemia.
Do not dilute or mix Lantus with any other insulin or solution. If mixed or diluted, the solution may become cloudy, and the onset of action/time to peak effect may be altered in an unpredictable manner. Do not administer Lantus via an insulin pump or intravenously because severe hypoglycemia can occur.
Hypoglycemia is the most common adverse reaction of insulin therapy, including Lantus, and may be life-threatening.
Hypoglycemia due to medication errors, such as accidental mix-ups between basal insulin products and other insulins, particularly rapid-acting insulins, have been reported. Patients should be instructed to always verify the insulin label before each injection.
Severe life-threatening, generalized allergy, including anaphylaxis, can occur. Discontinue Lantus, treat and monitor until symptoms resolve.
A reduction in the Lantus dose may be required in patients with renal or hepatic impairment.
As with all insulins, Lantus use can lead to life-threatening hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Closely monitor potassium levels in patients at risk of hypokalemia and treat if indicated.
Fluid retention, which may lead to or exacerbate heart failure, can occur with concomitant use of thiazolidinediones (TZDs) with insulin. These patients should be observed for signs and symptoms of heart failure. If heart failure occurs, dosage reduction or discontinuation of TZD must be considered.
Drug Interactions
Certain drugs may affect glucose metabolism, requiring insulin dose adjustment and close monitoring of blood glucose. The signs of hypoglycemia may be reduced in patients taking anti-adrenergic drugs (e.g., beta-blockers, clonidine, guanethidine, and reserpine).
Adverse Reactions
Adverse reactions commonly associated with Lantus include hypoglycemia, allergic reactions, injection site reactions, lipodystrophy, pruritus, rash, edema and weight gain.
Important Safety Information for Lantus SoloStar
Lantus SoloSTAR is a disposable single-patient-use prefilled insulin pen. To help ensure an accurate dose each time, patients should follow all steps in the Instruction Leaflet accompanying the pen: otherwise they may not get the correct amount of insulin, which may affect their blood glucose.
for Full Prescribing Information for Lantus.
for information on Sharps Medical Waste Disposal.
to learn more about Sanofi's commitment to fighting counterfeit drugs.
*Eligibility & Offer Terms For Your Patients:
Insulins Valyou Savings Program: This savings program is not insurance. For a complete list of participating brands, products, and National Drug Codes (NDCs) Click Here. This offer is not valid for prescriptions covered by or submitted for reimbursement, in whole or in part, under Medicare, Medicaid, VA, DOD, TRICARE, similar federal or state programs, including any state pharmaceutical programs. If you have an Affordable Care (Health Care Exchange) plan, you may still be qualified to receive and use this savings card. Please note: the Federal Employees Health Benefits (FEHB) Program is not a federal or state government health care program for purposes of the savings program. Void where prohibited by law. The Savings Program applies to the cost of medication. There are other relevant costs associated with overall treatment. Sanofi reserves the right to rescind, revoke, terminate, or amend this offer, eligibility, and terms of use at any time without notice. Upon registration, patients will receive all program details. For questions regarding your eligibility or benefits, or if you wish to discontinue your participation, call the Insulins Valyou Savings Program at (833) 813-0190 (8:00 am-8:00 pm EST, Monday-Friday).
Sanofi Insulins Co-pay Savings Program: This savings program is not insurance. For a complete list of participating brands, products, and National Drug Codes (NDCs) Click Here. This offer is not valid for prescriptions covered by or submitted for reimbursement, in whole or in part, under Medicare, Medicaid, VA, DOD, TRICARE, similar federal or state programs, including any state pharmaceutical programs. If you have an Affordable Care (Health Care Exchange) plan, you may still be qualified to receive and use this savings card. Please note: the Federal Employees Health Benefits (FEHB) Program is not a federal or state government health care program for purposes of the savings program. Void where prohibited by law. There are other relevant costs associated with overall treatment. Sanofi reserves the right to rescind, revoke, terminate, or amend this offer, eligibility, and terms of use at any time without notice. Upon registration, patients will receive all program details. For questions regarding your eligibility or benefits, or if you wish to discontinue your participation, call the Sanofi Insulins Co-pay Savings Program at (866) 255-8661 (8:00 am-8:00 pm EST, Monday-Friday).
REFERENCES:
- Lankisch MR, Ferlinz KC, Leahy JL, Scherbaum WA. Orals Plus Apidra® and Lantus® (OPAL) study group. Diabetes Obes Metab. 2008;10(12):1178-1185.
- Apidra® Prescribing Information.
- Philotheou A, Arslanian S, Blatniczky L, Peterkova V, Souhami E, Danne T. Diabetes Technol Ther. 2011;13(3):327-334.
- American Diabetes Association: Standards of medical care in diabetes—2006. Diabetes Care 2006;29(Suppl 1):S4–S42. Erratum in: Diabetes Care 2006;29:1192.
- American Diabetes Association Professional Practice Committee; 14. Children and Adolescents: Standards of Care in Diabetes—2024. Diabetes Care 1 January 2024; 47 (Supplement_1): S258–S281. https://doi.org/10.2337/dc24-S014.
- Daily G, et al. Diabetes Care. 2004;27:2363-2368.